|
|
|

|
Molecular Shapes
Copyright
D. Herrick |
 |
| |
20 Questions from past exams.
Practice for speed. Aim
for 2 minutes per problem.
|
|
| 1. |
The
shape of the ammonium ion NH4+ is |
|
| A) |
linear |
G) |
octahedral |
| B) |
V-shaped |
H) |
square
pyramidal |
| C) |
trigonal
planar |
I) |
square
planar |
| D) |
tetrahedral |
J) |
T-shaped |
| E) |
trigonal
pyramidal |
K) |
seesaw |
| F) |
trigonal
bipyramidal |
|
|
|
| 2. |
The
shape of the nitrate ion NO3- is |
|
| A) |
linear |
G) |
octahedral |
| B) |
V-shaped |
H) |
square
pyramidal |
| C) |
trigonal
planar |
I) |
square
planar |
| D) |
tetrahedral |
J) |
T-shaped |
| E) |
trigonal
pyramidal |
K) |
seesaw |
| F) |
trigonal
bipyramidal |
|
|
|
| 3. |
The
shape of [AlF6]3- is |
|
| A) |
linear |
G) |
octahedral |
| B) |
V-shaped |
H) |
square
pyramidal |
| C) |
trigonal
planar |
I) |
square
planar |
| D) |
tetrahedral |
J) |
T-shaped |
| E) |
trigonal
pyramidal |
K) |
seesaw |
| F) |
trigonal
bipyramidal |
|
|
|
| A) |
linear |
G) |
octahedral |
| B) |
V-shaped |
H) |
square
pyramidal |
| C) |
trigonal
planar |
I) |
square
planar |
| D) |
tetrahedral |
J) |
T-shaped |
| E) |
trigonal
pyramidal |
K) |
seesaw |
| F) |
trigonal
bipyramidal |
|
|
|
| A) |
linear |
G) |
octahedral |
| B) |
V-shaped |
H) |
square
pyramidal |
| C) |
trigonal
planar |
I) |
square
planar |
| D) |
tetrahedral |
J) |
T-shaped |
| E) |
trigonal
pyramidal |
K) |
seesaw |
| F) |
trigonal
bipyramidal |
|
|
|
|
Complete
the Lewis structure for questions 6-9: |
|
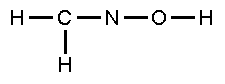 |
|
| 6. |
The
C-N bonding consists of |
|
| A) |
one sigma bond |
| B) |
one
pi bond |
| C) |
two
sigma bonds |
| D) |
one
sigma bond and one pi bond |
| E) |
two
pi bonds |
| F) |
two
sigma bonds and one pi bond |
| G) |
one
sigma bond and two pi bonds |
|
| 7. |
The
N-O-H bond angle is |
|
| A)
90° |
B)
109.5° |
C)
120° |
D)
150° |
E)
180° |
|
| 8. |
The
C-N-O bond angle is |
|
| A)
90° |
B)
109.5° |
C)
120° |
D)
150° |
E)
180° |
|
| 9. |
The
valence bonding between N and O involves overlap of
the atomic orbitals |
|
| A) |
2s
on N and 2s on O |
F) |
sp2
on N and sp3 on O |
| B) |
2p
on N and 2s on O |
G) |
sp3
on N and sp2 on O |
| C) |
2s
on N and 2p on O |
H) |
sp3
on N and sp3 on O |
| D) |
2p
on N and 2p on O |
I) |
sp
on N and sp3 on O |
| E) |
sp2
on N and sp2 on O |
|
|
|
| 10. |
What
are the shape and the overall polarity of OF2
? |
|
| A) |
bent
(109.5°), polar |
| B) |
bent
(109.5°), nonpolar |
| C) |
bent
(120°), nonpolar |
| D) |
bent
(120°), polar |
| E) |
linear
(180°), nonpolar |
|
| 11. |
| The figure depicts a set of ____
hybrid orbitals. |
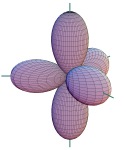
|
|
|
| A) sp3 |
B)
sp2d |
C)
p2d3 |
D)
sp3d |
E)
sp2d2 |
|
| 12. |
How many p orbitals participate in the formation of
orbitals with tetrahedral hybridization?
|
|
| 13. |
The
alignment of atomic orbitals on different atoms for
pi
bonding between the atoms is
|
|
| 14. |
Which
bonds restrict rotation of a molecular group about the bond
axis?
1 = sigma bond, 2 = pi bond
|
|
| A) |
1
only |
| B) |
2
only |
| C) |
1
and 2 |
| D) |
none |
|
| 15. |
| Neglecting
any possible delocalization, the bonding "5" is best
described as |
Questions
15-17: trans-butadiene
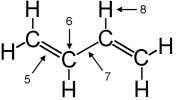 |
|
|
| A) |
two
sigma bonds |
| B) |
two
pi bonds |
| C) |
one
sigma bond and one pi bond |
| D) |
one
sigma bond and two pi bonds |
| E) |
two
sigma bonds and one pi bond |
|
| 16. |
The hybridization at carbon atom "6" is
|
|
| A)
s2p2 |
B)
sp2 |
C)
sp3 |
D)
s2p
|
E)
p3 |
|
| 17. |
Including
delocalization, the bonding "7" is best described as |
|
| A) |
a
pure sigma bond |
| B) |
a
pure pi bond |
| C) |
a
sigma bond plus partial pi bonding |
| D) |
a
pi bond plus partial sigma bonding |
| E) |
one
sigma bond plus one pi bond |
|
| 18. |
The
bonding between C and N in HCN is best described
as
|
|
| A) |
one
sigma bond and one pi bond |
| B) |
one
sigma bond and two pi bonds |
| C) |
two
sigma bonds |
| D) |
two
sigma bonds and one pi bond |
| E) |
three
sigma bonds |
| F) |
three
pi bonds |
|
| 19. |
The
expected O-S-O bond angle in the sulfate ion is |
|
| A)
90° |
B)
109.5° |
C)
120° |
D)
150° |
E)
180° |
|
| 20. |
The
expected Cl-I-Cl bond angle in ICl2-
is
|
|
| A)
90° |
B)
109.5° |
C)
120° |
D)
150° |
E)
180° |
|
|
|
|